Feature 1
Sales, Technical and Clinical Support
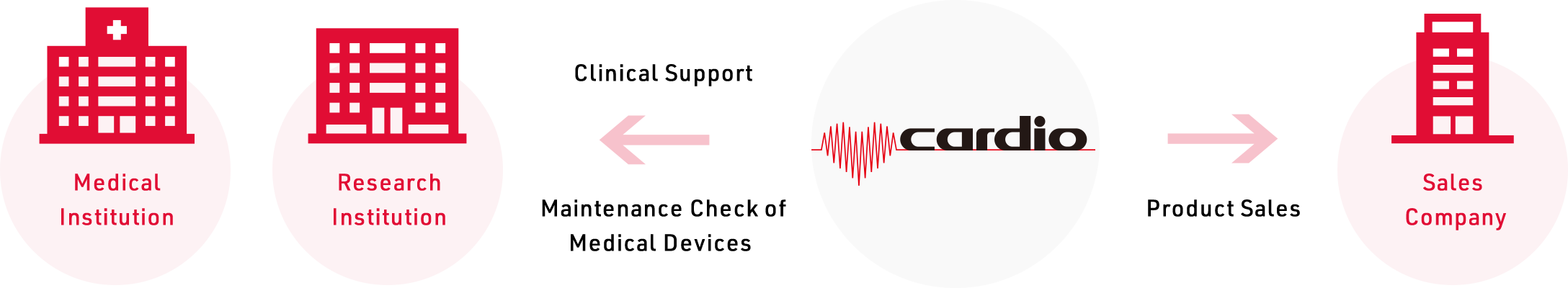
Cardio Inc. is an independent medical device import and sales company that is not affiliated with any medical device manufacturer, therefore we can import and sell medical devices with our own intention. We are not just a sales agent. We handle everything from market approval to sales and marketing, clinical support as well.
We have an experienced staff of qualified pharmacists and clinical engineers who provide clinical support to promote the medical devices safely. We hold regular workshops and users meetings to deepen users’ knowledge of medical devices and build relationships of trust with users.
We also have the medical device maintenance and inspection facility and the technical team.
By having the technologies and knowledge about the products by regular training from medical device manufacturers and communicate with them as needed to constantly update their knowledge of medical device technologies, we can provide useful information and advice to users and respond to clinical and product technology.
Feature 2
Clinical trial and Launch into Japanese market
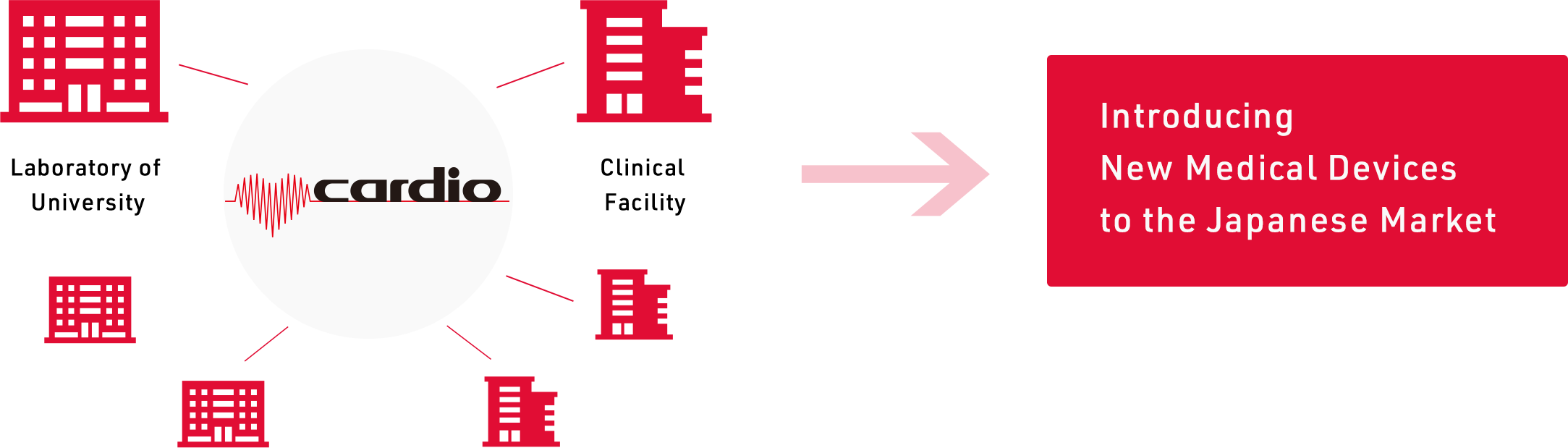
Cardio Inc. has experience of introducing Class IV products (see " Imported Products ") into Japanese Market.
Utilizing this experience, we can propose and promote the acquisition of market approval efficiently, including the necessity of clinical trials and the scale of clinical trials, taking into consideration the scale of sales.
Even if products were previously considered to be difficult to introduce into the Japanese market due to their small market size, we may be able to propose from different perspectives.
Feature 3
Business Licences and QMS
There are four classes of medical devices in Japan: Class I (general medical devices), Class II (Controlled medical devices), Class III, and IV (Specially-controlled medical devices). Depending on the medical device class, the first to third class marketing license for medical devices is required.
| Criteria for license | Criteria for medical devices | Classifications |
|---|---|---|
| First-class marketing license for medical devices | Specially-controlled medical devices | Class IV |
| Class III | ||
| Second-class marketing license for medical devices | Controlled medical devices | Class II |
| Third-class marketing license for medical devices | General medical devices | Class I |
*Red text is the license owned by Cardio Inc.
Cardio Inc. currently handles Class I to IV medical devices, and has the first-class marketing license for medical devices and the Quality Management System (QMS) .
We have some experiences of obtaining the approval for marketing authorization for overseas advanced medical devices, and launching them to the Japanese market, and promoting commercialization.



