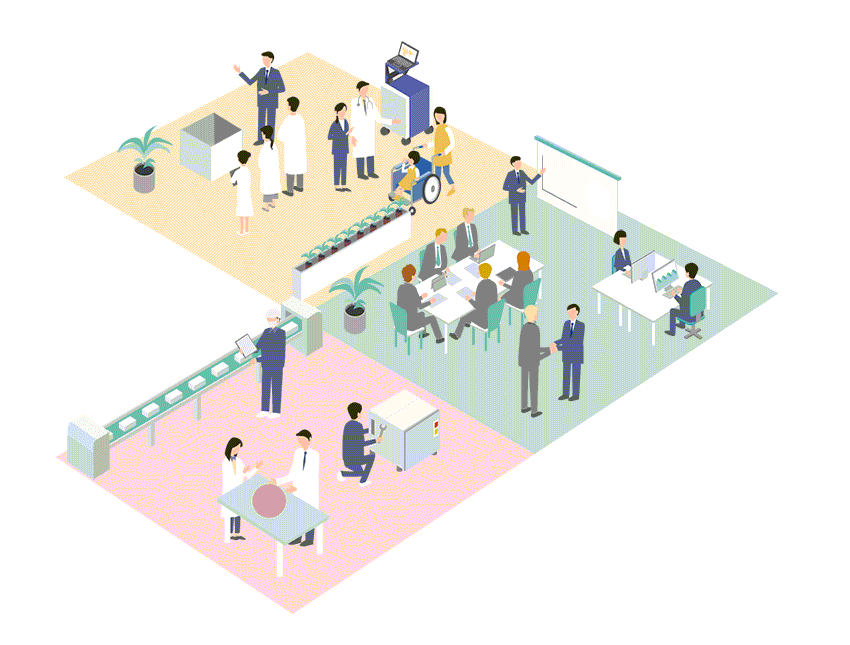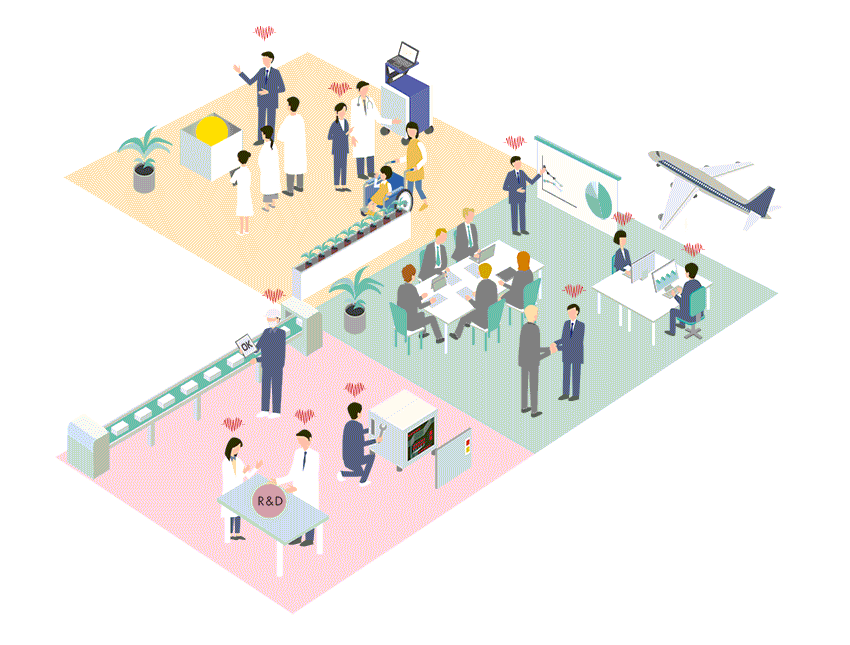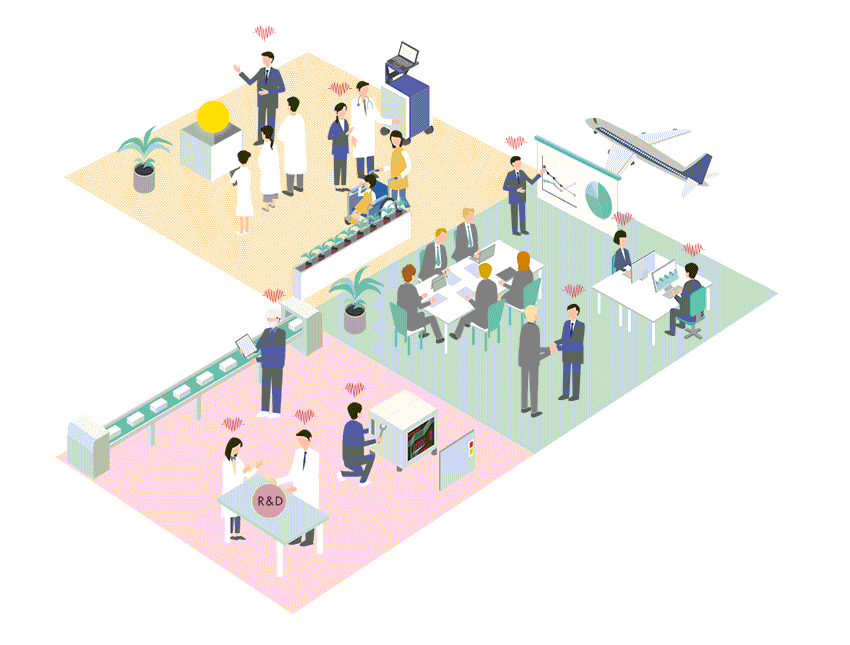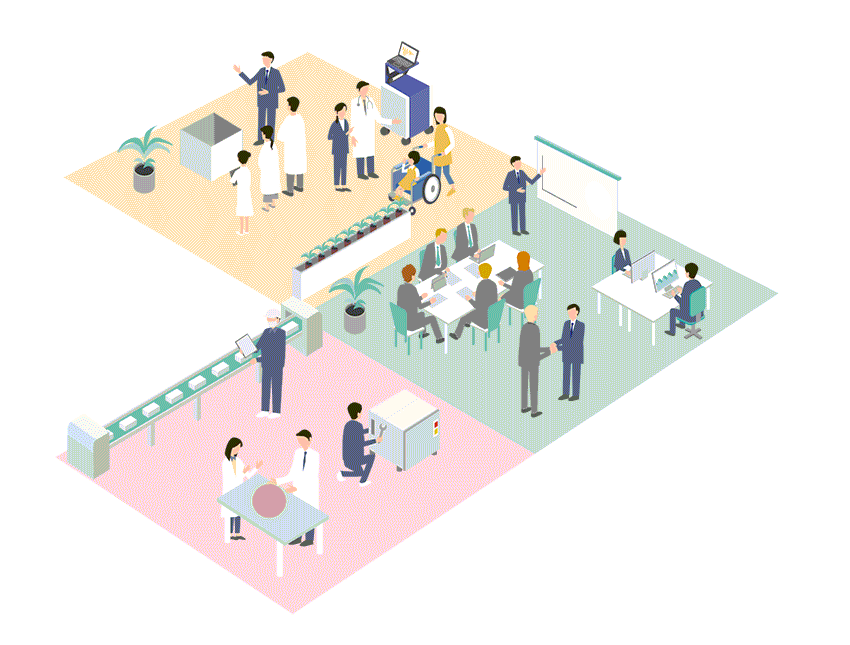
Business Policy
Regardless of the size of the market, Cardio Inc. seeks out advanced, unique and outstanding medical technologies and devices from all over the world, and builds business in Japan and Asia.
Cardio Inc. has created an efficient business structure with a small number of expert staff. Therefore, even for small markets that are considered difficult to commercialize, if it is original and useful for patient treatment and QOL improvement, we will propose marketing and business strategies and develop the market.
Business developments of medical devices in Japan and Asia
Cardio Inc. started as a research and development company for regenerative medicine, and has grown by holding marketing authorization for medical devices. Thanks to this unique structure and history of the company, we have strong ties with research facilities, researchers and distributors not only in Japan but also overseas. By effectively utilizing these networks, we collect information on cutting-edge technologies and medical devices from around the world, and if we judge the product is highly useful, we will start negotiations with the manufacturer from an early stage. We not only aim to achieve a quick launch in Japan, but also expand the scope to Asia.
Cardio Inc. will handle obtaining market approval for medical devices that are beneficial for patients, even if other import and sales companies hesitate to do so because of regulatory difficulties.
Clinical Support of medical devices in Japan
In order to increase sales and expand the market size safely, clinical support is provided by Cardio Inc.’s experienced staff who are qualified as pharmacists and clinical engineers. In addition, in partnership with academic societies and clinical facilities, we hold regular workshops and users meetings to deepen users’ knowledge of medical devices and build relationships of trust with users.
Technical Support of medical devices
By owning maintenance and inspection facilities for medical devices and with our technical team, Cardio Inc. is constantly improving its knowledge of medical devices and their safe usage.
Skilled technicians receive regular training from medical device manufacturers and keep in touch with them as needed to constantly update their knowledge of medical device technology. We have also built a system for providing appropriate technical support to users.
Business licenses and QMS
Cardio Inc. has a first-class marketing license for medical device and Quality Management System (QMS). We obtain approval for marketing authorization for advanced medical devices in Japan and overseas to promote introduction and commercialization in the market.
The professional pharmaceutical department, which is in charge of approval application, corresponding with the Ministry of Health, Labour and Welfare, and QMS operation, will draw up an optimal plan for obtaining market approval, and also handle inquiries, correspondence and post-marketing monitoring.
Using its achievements and experience of self-developed products, clinical trials of class IV products and market approvals, Cardio Inc. proposes efficient and speedy strategies in line with the Pharmaceutical and Medical Device Act and in consideration of market size and product characteristics.